▼▼▼▼▼▼▼▼▼▼▼▼
|
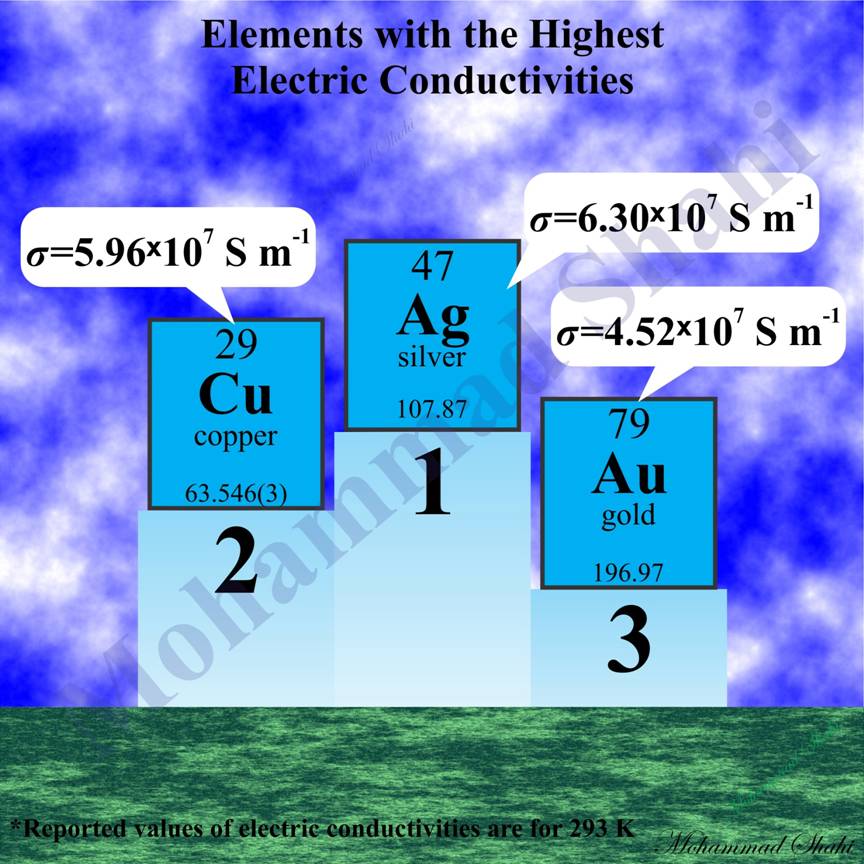
Elements With the Highest Electric Conductivities
Resistivity (symbol: ρ) is defined as electric field strength, E, divided by the current density, j (This definition is from IUPAC gold book). For an ideal case (where E=V/d, j=I/A and R=V/I), this is equivalent to the following relationship: ρ = RA/l Where R is the electrical resistance, l is the length and A is the cross-sectional area. The SI unit of electric resistivity is ohm-meter (Ω.m). Electric resistivity is a measure of opposition to the flow of electric current, e.g. electric current passes a material with lower electric resistivity more easily than a material with higher electric resistivity. Electric conductivity (symbol: γ or σ) is the reciprocal of resistivity (σ=1/ρ). Electric conductivity is a measure of ability to conduct an electric current, e. g. materials with higher electric conductivities are better electrical conductors. The SI unit of electric conductivity is siemens per meter (S/m) where one siemens is equal to the reciprocal of one ohm (1 S = 1 Ω-1).† ††
Among elements and alloys, silver (Ag), copper (Cu) and gold (Au), elements of the group 11, are famous to have the highest electric conductivities. As a result, they are the best choices for making electrical conductors like electrical cables. Silver has higher electric conductivity than copper but its price is too high which makes it unsuitable for extensive use. As a result, copper is the metal that is widely used in electrical conductors although it has some competitors like aluminium which arenít as good as copper in the value of electric conductivity but may give better results for other parameters of concern like the ratio of electric conductivity to mass (weight). More than half of mined copper is used to manufacture electrical conductors where copper is mostly used in its pure form to prevent reduction of electric conductivity by impurities.
|


