▼▼▼▼▼▼▼▼▼▼▼▼
|
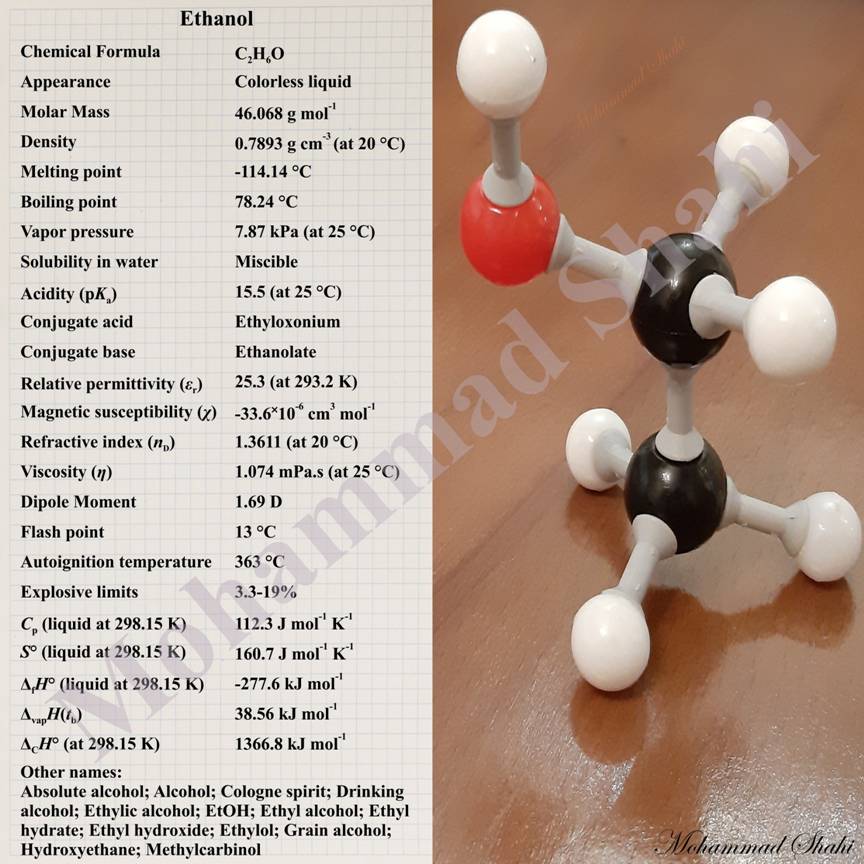
Ethanol
Ethanol with the molecular formula C2H5OH is one of the simplest alcohols. It consists of a hydroxy group, —OH, and an ethyl group, —CH2CH3, attached together. Based on its structure, it can be abbreviated as EtOH. Ethanol is a colorless liquid at room temperature and pressure. Ethanol is considered to be relatively volatile although it can have hydrogen bonds between its molecules which strengthen its intermolecular interactions considerably and result in much higher values for physical properties like its melting and boiling points in comparison to those of many other organic and inorganic substances with similar molecular weights. For example, while ethanol (Mr(CH3CH2OH)≈46) boils at about 78 °C, propane (Mr(CH3CH2CH3)≈44) and dimethyl ether (Mr(CH3OCH3)≈46), two examples of substances with similar molecular weights but no hydrogen bonds between their molecules, respectively boil at -42 °C and -24 °C. Ethanol has a distinctive odor similar to that of methanol.
Beside its famous use as a popular psychoactive substance and recreational drug, ethanol has many other applications like consumption as an antiseptic and disinfectant in medicine, usage as a widely-used solvent in laboratories and industry, use as a precursor for other commodity chemicals like ethyl esters and also consumption as a clean-burning fuel. Industrially ethanol is primarily produced either by acid-catalyzed hydration of petrochemical ethylene (C2H4+H2O—>CH3CH2OH) or by fermentation of sugars in grains with yeast (which can be summarized as C6H12O6—>2CH3CH2OH+2CO2 and C12H22O11+H2O—>4CH3CH2OH+4CO2). It may be interesting to know that the fermentation of sugar into ethanol and its consumption for intoxication by human may date back 10,000 years.
|


