▼▼▼▼▼▼▼▼▼▼▼▼
|
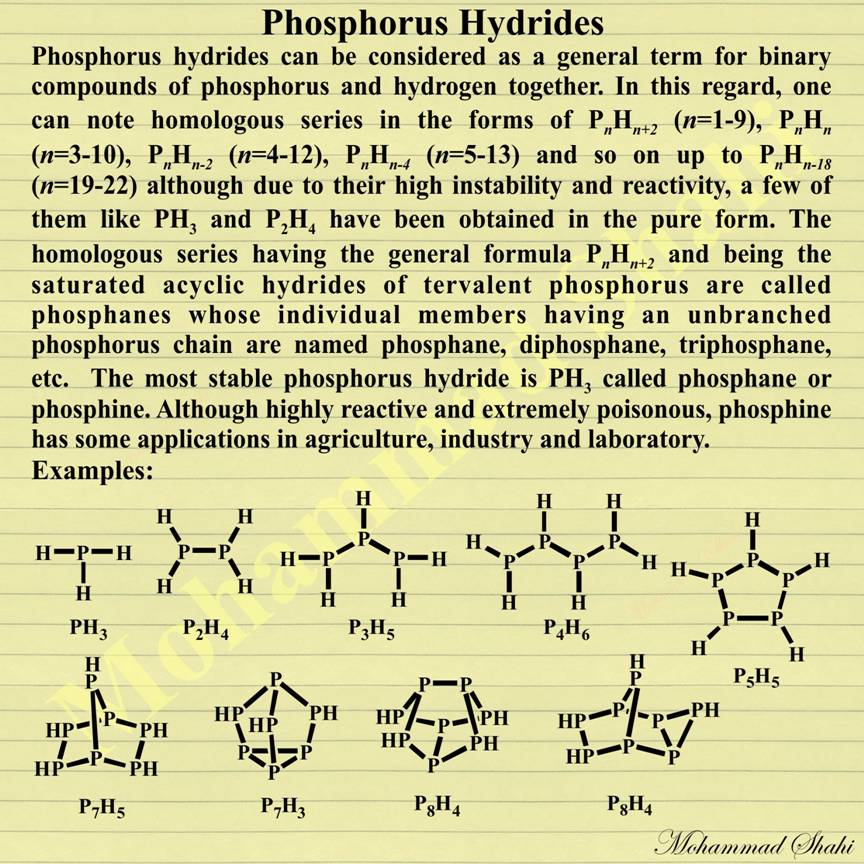
Phosphorus Hydrides
Phosphorus hydrides are binary compounds of phosphorus and hydrogen together. In addition to relatively famous PH3 and P2H4, some other phosphorus hydrides also exist in the forms of PnHn+2 (n=1-9), PnHn (n=3-10), PnHn-2 (n=4-12), PnHn-4 (n=5-13) and so on up to PnHn-18 (n=19-22). They are very unstable and reactive (even the most stable one, PH3, is highly reactive). Among phosphorus hydrides, phosphanes are the saturated acyclic hydrides of tervalent phosphorus with the general formula PnHn+2 including phosphane(PH3), diphosphane (P2H4), triphosphane (P3H5), etc.
|


