▼▼▼▼▼▼▼▼▼▼▼▼
|
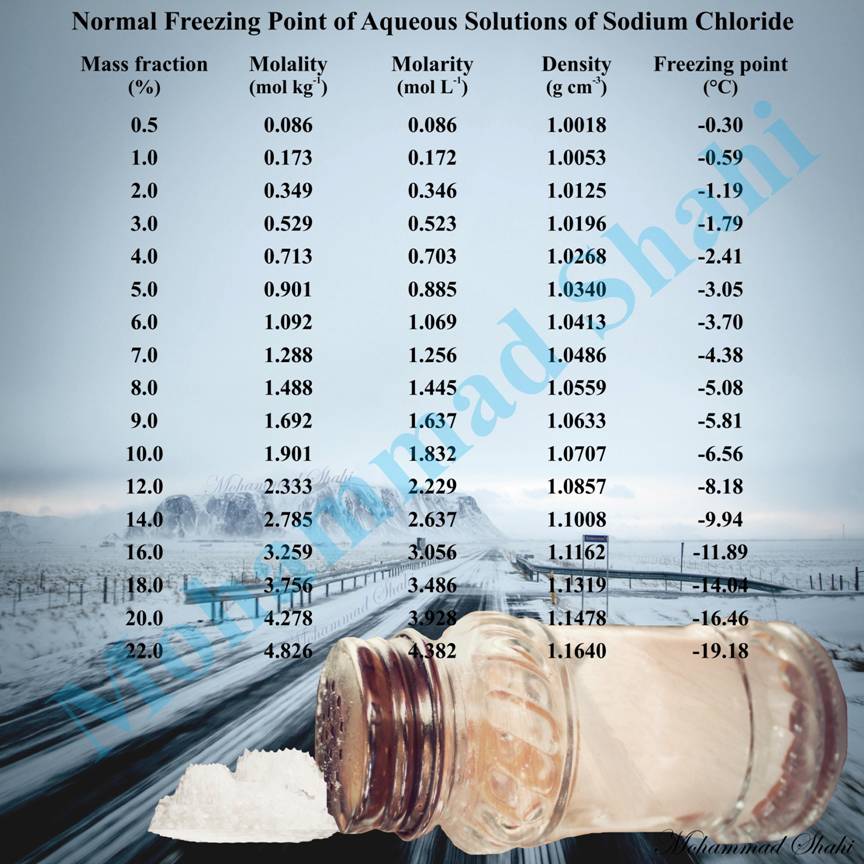
Normal Freezing Point of Aqueous Solutions of Sodium Chloride
Sodium chloride also known as salt has many other applications beside its familiar domestic uses. Its two major applications are its direct or indirect use in the production of many chemicals and de-icing of roads in winter. Sodium chloride lowers the freezing point of water and as a result, when it is applied on roads, it acts as an anti-icing or de-icing agent. It is one of the cheapest and easiest ways to deal with icy road in winter however its use is associated with environmental problems and has caused concerns among scientists and environmentalists.
|


