▼▼▼▼▼▼▼▼▼▼▼▼
|
Platonic Hydrocarbons
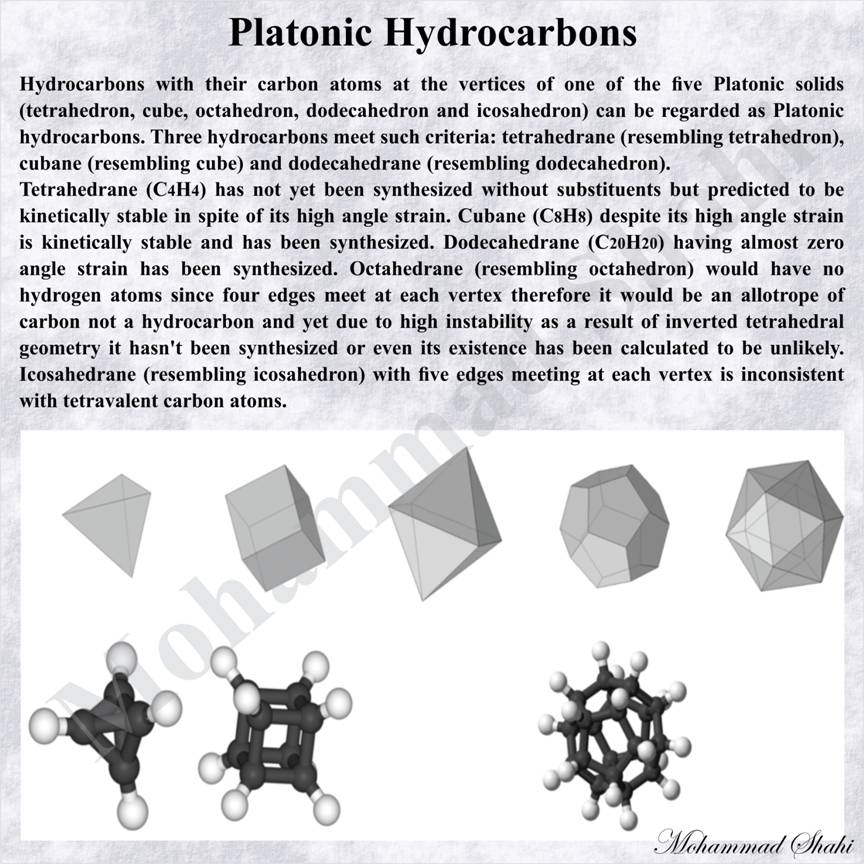
Hydrocarbons with their carbon atoms at the vertices of one of the five Platonic solids (tetrahedron, cube, octahedron, dodecahedron and icosahedron) can be regarded as Platonic hydrocarbons. Three hydrocarbons meet such criteria: tetrahedrane (resembling tetrahedron), cubane (resembling cube) and dodecahedrane (resembling dodecahedron).
Tetrahedrane (C4H4) has not yet been synthesized without substituents but predicted to be kinetically stable in spite of its high angle strain. Cubane (C8H8) despite its high angle strain is kinetically stable and has been synthesized. Dodecahedrane (C20H20) having almost zero angle strain has been synthesized. Octahedrane (resembling octahedron) would have no hydrogen atoms since four edges meet at each vertex therefore it would be an allotrope of carbon not a hydrocarbon and yet due to high instability as a result of inverted tetrahedral geometry it hasnít been synthesized or even its existence has been calculated to be unlikely. Icosahedrane (resembling icosahedron) with five edges meeting at each vertex is inconsistent with tetravalent carbon atoms.
|


