▼▼▼▼▼▼▼▼▼▼▼▼
|
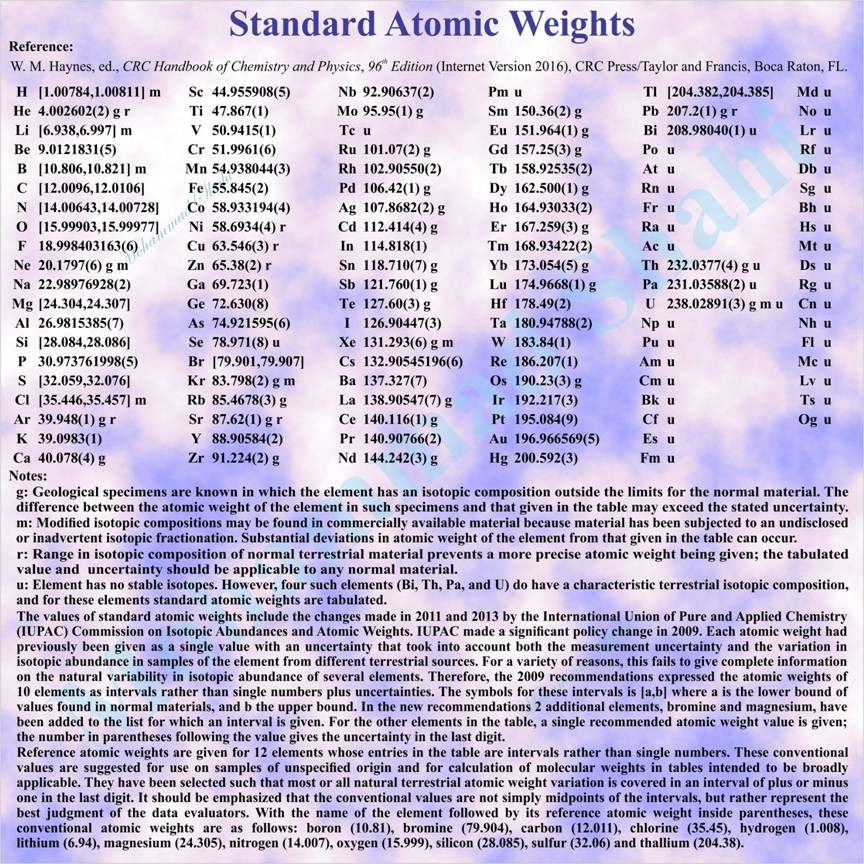
Standard Atomic Weights (2013)
Standard atomic weights are recommended values of relative atomic masses of the elements revised biennially by the IUPAC Commission on Atomic Weights and Isotopic Abundances and applicable to elements in any normal sample with a high level of confidence. A normal sample is any reasonably possible source of the element or its compounds in commerce for industry and science and has not been subject to significant modification of isotopic composition within a geologically brief period. (Source: IUPAC Gold Book)
Relative atomic mass or atomic weight is the ratio of the average mass of the atom to the unified atomic mass unit where unified atomic mass unit (symbol: u) is non-SI unit of mass equal to the atomic mass constant defined as one twelfth of the mass of a carbon-12 atom in its ground state and used to express masses of atomic particles (u is approximately equal to 1.6605402(10)×10^-27 kg and is also equal to dalton (symbol: Da), non-SI unit of mass often used in biochemistry and molecular biology). The recommended symbol of relative atomic mass is Ar where A is printed in italic (sloping) type and modified by the subscript r printed in Roman (upright) type.
For elements with no stable isotopes denoted by u in above table, individual isotopic masses can be found in other tables.
|


