▼▼▼▼▼▼▼▼▼▼▼▼
|
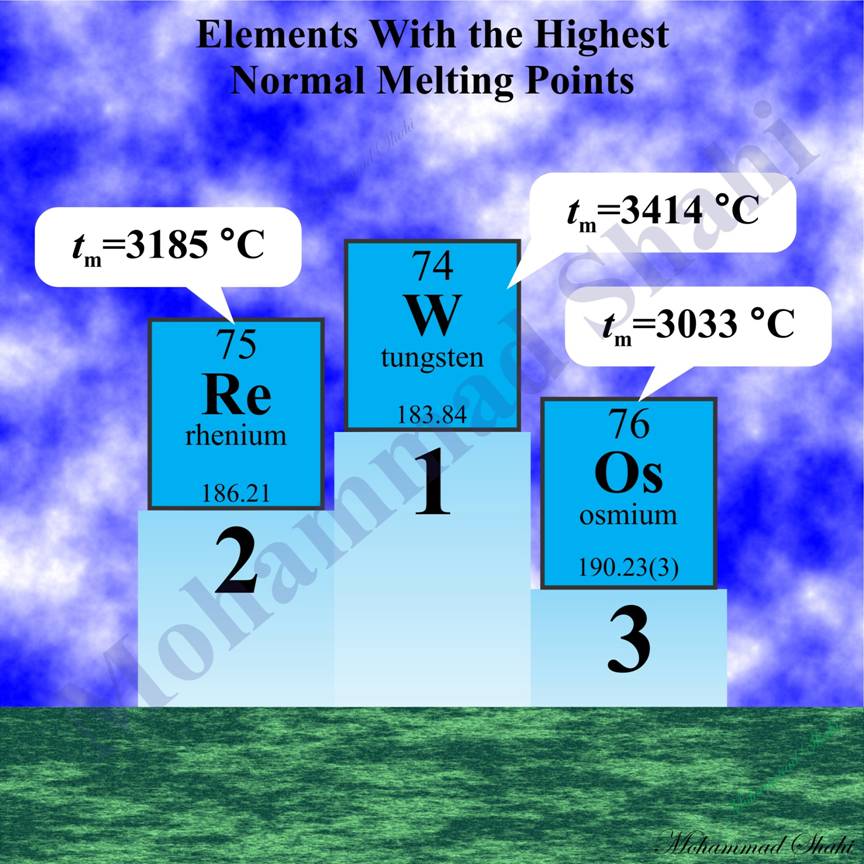
Elements With the Highest Normal Melting Points
Tungsten (W, Z=74), rhenium (Re, Z=75), osmium (Os, Z=76) and tantalum (Ta, Z=73) have the highest normal melting and boiling points among metals and also elements. For these elements, tabulated values of normal melting points in degrees Celsius reported in CRC handbook (96th) are as follows: W (3414 °C), Re (3185 °C), Os (3033 °C) and Ta (3017 °C). For normal boiling points, we have: Re (5590 °C), W (5555 °C), Ta (5455 °C) and Os (5008 °C).
Based on reported values in different handbooks, tungsten and rhenium have the 1st and 2nd ranks in normal boiling point (bp) but different handbooks report different values for bp of these two metals especially in the case of tungsten (for example 5930 °C instead of 5555 °C). As a result, based on values of handbooks like CRC, rhenium has the highest bp while based on values of some other handbooks, the highest bp belongs to tungsten.
It is also good to mention that famous diamond and graphite (allotropes of carbon) have no normal melting point. Based on CRC handbook, the melting point of diamond at the pressure of 12.4 GPa is 4440 °C. The triple point of graphite is 4489 °C at the pressure of 10.3 MPa. In addition, the sublimation point of graphite is 3825 °C at which the pressure of vapor in equilibrium with the solid reaches 101.325 kPa.
|


