▼▼▼▼▼▼▼▼▼▼▼▼
|
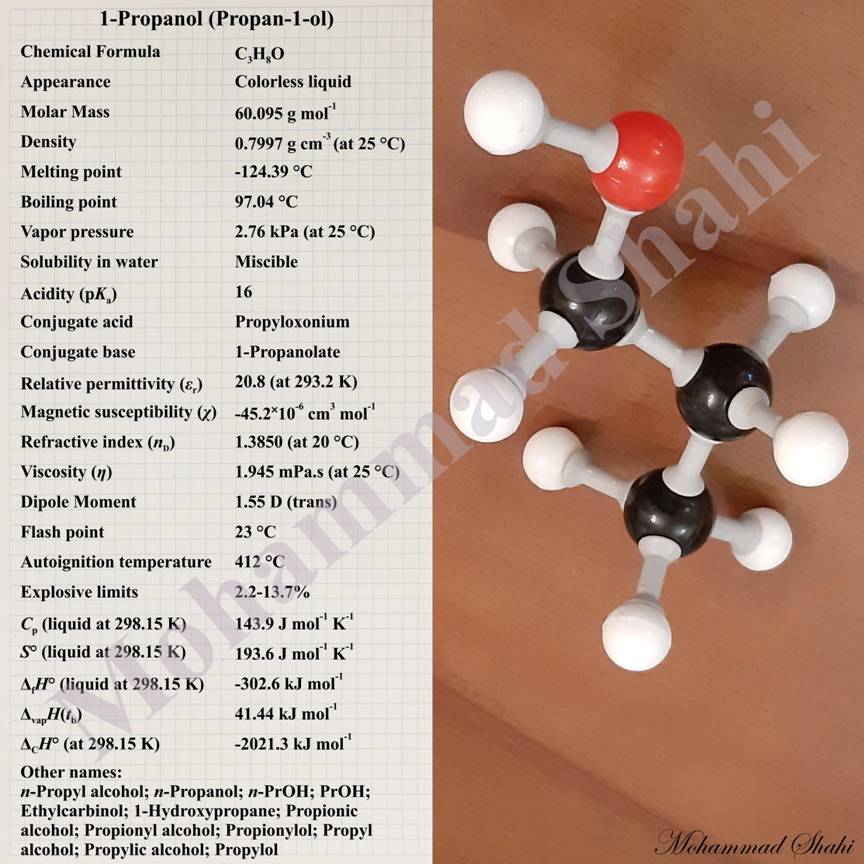
1-Propanol
Propan-1-ol or 1-propanol also known as n-propanol, n-propyl alcohol, propionic alcohol and propylol has the chemical formula CH3CH2CH2OH. Like other alcohols, it has a hydroxy group, —OH, attached to a saturated carbon atom. It can be abbreviated as PrOH or (n-PrOH). 1-Propanol is a colorless liquid at room temperature and pressure. Like other alcohols, it can have hydrogen bonds between its molecules which strengthen its intermolecular interactions and result in much higher values of physical properties like melting and boiling points in comparison to many other organic and inorganic substances with similar molecular weights. For example, while propan-1-ol boils at 97 °C, methoxyethane (CH3OCH2CH3) with the same molecular weight but no hydrogen bonds between its molecules boils at 7.4 °C. Like other alcohols, propan-1-ol has both a hydrophilic (literally water loving) group, which is the hydroxy group (—OH), and a hydrophobic (lipophilic or literally fat loving) group which is the propyl group (—CH2CH2CH3).
In addition to 1-propanol, another alcohol, 2-propanol or propan-2-ol, exists with the molecular formula of C3H8O which has its hydroxy group attached to the middle carbon (it has the form CH3CHOHCH3). These two alcohols, propan-1-ol and propan-2-ol, in a broad sense are constitutional (structural) isomers of each other. In a more specific way, they are position isomers (positional isomers) of each other. One should note that by constitutional isomerism we mean isomerism between structures differing in constitution like CH3CH2CH2OH, CH3CHOHCH3 and CH3CH2OCH3 as constitutional isomers of each other; while by position isomerism (regioisomerism) we mean isomerism between structures differing in the position of a functional group or substituent on a same parent structure like CH3CH2CH2OH and CH3CHOHCH3 as positional isomers of each other. Position isomerism can be considered as a specific form of constitutional isomerism.
Propan-1-ol has some applications like its use as a solvent in the pharmaceutical industry. It is industrially produced by catalytic hydrogenation of propanal (CH3CH2CH=O + H2 —> CH3CH2CH2OH) where propanal itself is obtained from the hydroformylation or oxo reaction of ethylene, carbon monoxide and hydrogen together (H2C=CH2 + CO + H2 —> CH3CH2CH=O). Propan-1-ol can also be produced naturally in small amounts by fermentation processes. Considering its octane number and anti-knock index, propan-1-ol is suitable for engine fuel usage which is hindered by its price.
|


