▼▼▼▼▼▼▼▼▼▼▼▼
|
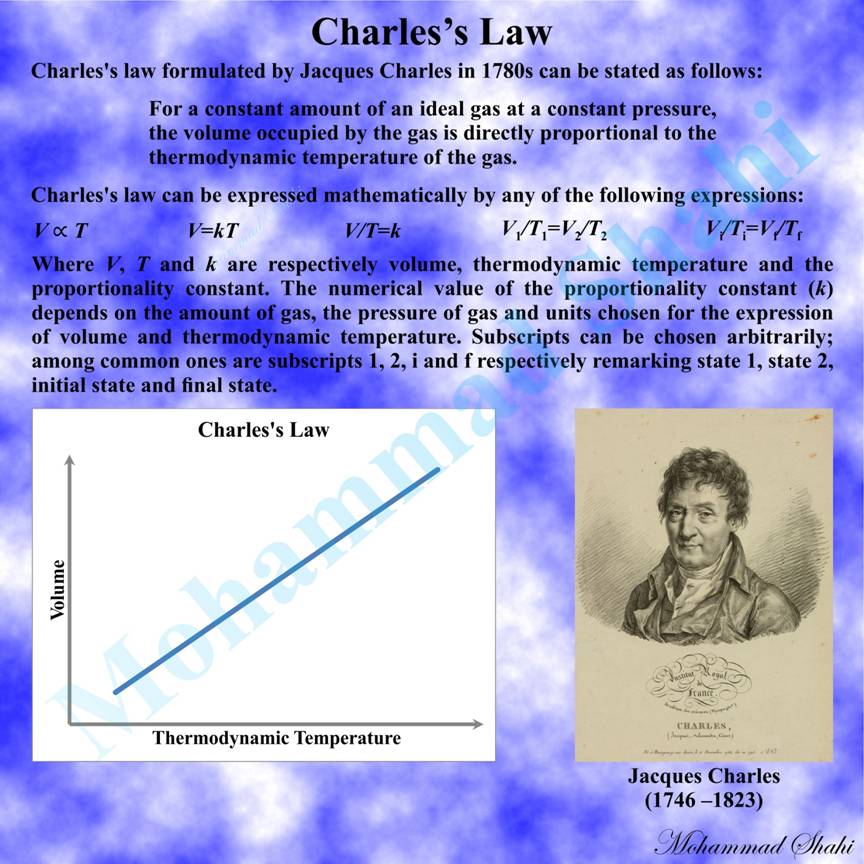
Charles’s Law
Charles’s law, originally formulated by the scientist Jacques Charles in his unpublished work from 1780s, addresses the proportional relationship between the volume and thermodynamic temperature of a gas under controlled conditions. It can be stated as follows: For a constant amount of an ideal gas at a constant pressure, the volume occupied by the gas is directly proportional to the thermodynamic temperature of the gas.
|


